By: Lauren Hammond
Leticia Shea, PharmD, BCACP
Robin Wackernah, PharmD, BCPP
Matt Fete, PhD
Abstract
The use of Cannabis is extensive and dynamic, dependent not only on the strain of the plant, but also on the formulation and the vehicle used for administration. Several different constituents of the plant, known as phytocannabinoids, have been studied for numerous conditions, including amyotrophic lateral sclerosis, multiple sclerosis, schizophrenia, anxiety disorders and for the use in pain management.1,2,3 This article aims to provide a review of the research findings for the use of marijuana in epilepsy, including the phytocannabinoids with the most evidence shown to inhibit or diminish seizure propagation. In addition, the pathways that determine formulations for administration and a review of drug resistant epilepsy will be discussed.
Introduction
There are numerous medications and treatment options for epilepsy that have been researched in clinical trials and brought to the market in the standard, evidenced-based medicine format. Regardless, many patients with epilepsy remain resistant to treatment options and seek additional therapies for better management of their condition. In the United States it is determined that of the 3 million people that live with epilepsy, 1 million are resistant to treatment and present with uncontrolled seizures regardless of their current medication therapy.4 Many medications approved for epilepsy come with intolerable side effects that may hinder patient compliance. Marijuana research is lacking well designed clinical trials as a result of local and federal regulation. This, however, has not stopped in-vitro and in-vivo studies. In the 1980’s two double-blinded studies with small samples sizes examined the use the phytocannabinoid cannabidiol (CBD), in the treatment of epilepsy.5,6 Although CBD has demonstrated anticonvulsant properties, conflicting studies suggest that other phytocannabinoids in Cannabis provide a synergistic antiepileptic benefit that cannot be achieved with CBD alone. Much of the data to support the use of marijuana in the treatment of epilepsy is based on research examining signaling pathways where different active moieties of Cannabis target, modulate and interfere with signaling responsible for seizure propagation. These initial findings provide foundational support for continued research to further determine and examine potential therapeutic benefits of marijuana in the treatment of epilepsy.
Epilepsy
Epilepsy is a broad term encompassing numerous syndromes that feature unprovoked seizure activity. The type of seizure, origination and progression can vary and the exact mechanism(s) are not fully understood. It is generally accepted that seizures occur as a result of excessive cerebral neuron discharge.7 Oscillations in excitatory activity within the brain have also been determined to be a likely factor in particular types of seizure induction.8 Phytocannabinoids such as delta (9)-tetrahydrocannabinol (THC) and CBD interact with receptors that may inhibit excitatory activity and also prevent the breakdown of endogenous cannabinoids, (endocannabinoids), which may further prevent excessive activity.
Drug Resistant Epilepsy
Drug resistant epilepsy (DRE) is defined as the inability to achieve complete seizure elimination after an adequate trial of two AEDs at therapeutic doses when used as either monotherapy or in conjunction with other anti-epileptic drugs (AEDs).9 In accordance with this definition, approximately 30-35% of patients meet the criteria for DRE.10 A population based study in Singapore reported 21.5% of patients having DRE.11
While the exact cause of DRE has not been fully elucidated, there several mechanisms proposed. Theories include increased p-glycoprotein expression12, alterations in gamma-aminobutyric acid A (GABA) expression13, genetic polymorphisms14 and cell death secondary to mitochondrial dysfunction.15
DRE impacts a patient’s quality of life, specifically; it is associated with reduced cognition, reduced energy, altered social functioning and an overall lowered emotional well-being.16 Additionally, patients with DRE may have an increased risk of death. High seizure frequency, multiple AEDs and years with epilepsy are potential theories that may clarify the causes of sudden unexplained death in epilepsy.17 Although not strongly demonstrated, death secondary to cardiac conduction abnormalities in DRE has also been reported.18 This reduced quality of life and increased chance of mortality emphasizes the need for medications that utilize novel mechanistic targets. Currently, the primary mechanisms for AEDs focus on sodium or calcium channels and enhancement of GABA or inhibition of glutamate. In addition to Cannabis, glial cells19, brain derived neurotrophic factor20 interleukin-1 beta receptor caspase 1 inhibitors21 are all potential targets for the treatment of DRE.
Endocannabinoid system
The endocannabinoid system (ECS) is comprised of endogenous lipid ligands, endocannabinoids, and their receptors. It has numerous effects on the body and there remains opportunity for discovery of additional biological effects. The main receptors of interest are G-protein-coupled cannabinoid-1 (CB1R) and cannabinoid-2 (CB2R). Our review focuses on CB1R due to its effects on neuronal transmission and how this may play a role in the prevention of seizure induction and/or propagation. CB2R is generally associated with immune function and expressed only in the periphery.22,23
CB1R is the most predominant of the two receptors and is largely expressed in the brain (substantia nigra, globus pallidus, hippocampus, cerebral cortex, putamen, caudate, cerebellum, and amygdala), with limited expression in adipose and hepatocyte tissue.22 CB1R can be activated by endogenous or exogenous cannabinoids.24 Endogenous cannabinoids, known as endocannabinoids, include anandamide (AEA) and 2-arachidonoyl glycerol (2-AG). AEA and 2-AG are responsible for the regulation of the endocannabinoid system. They are made on demand, from increased neuronal excitability.25 Fatty acid amide hydrolase (FAAH) catabolizes AEA and monacylglycerol lipase degrades 2-AG. These are all important modulators of regulation in the ECS.22 Neuronal excitability triggers the release of endocannabinoids into the synaptic cleft from the postsynaptic neuron where they bind to presynaptic neuron CB1R resulting in inhibition of neurotransmitter release at both excitatory and inhibitory synapses.26,27 This inhibits the release of either glutamate or GABA.28 Evidence suggests that activation of CB1R on glutamatergic rather than GABAergic terminals is required for antiepileptic action of endogenous cannabinoids.29 This brief discussion of the ECS highlights the complexity of these poorly understood pathways. Research has shown that exogenous cannabinoids that interact at these receptors, including THC and CBD, provide distinctly different responses, and yet both have been shown to provide benefit in inhibiting or slowing seizure propagation. It is beyond the scope of this review to analyze all of the mechanisms responsible for Cannabis ’activity. Our goal is to highlight the complexity of the various cannabinoids found within Cannabis and summarize what research has been done with these cannabinoids in relation it their use in epilepsy.
Endocannabinoids Studied for the Use in Epilepsy
The two most common phytocannabinoids in marijuana are THC and CBD. Both compounds, which can be derived from either strain of marijuana, Cannabis sativa or Cannabis indica, have been shown to have anticonvulsant and antiepileptic properties.22 Of the many phytocannabinoids present in Cannabis, THC and CBD are the 2 constituents with research to support their use in epilepsy. THC is the major psychoactive component of marijuana whereas CBD is the primary nonpsychoactive component. The interaction and effect when THC binds to CB1R (and CB2R) is dramatically different than that of CBD.30 THC binds to both CB1R and CB2R with relatively high affinity but lower than known synthetic CB1/CB2 receptor agonist WIN 55,212-2. WIN 55, 212-2 is a synthetic CB1/CB2 agonist which has been studied to evaluate the numerous effects of binding to cannabinoid receptors.28 THC resembles AEA affinity for CB1R but has lower efficacy than the synthetic agonists, suggesting THC to be a partial agonist.28 There is evidence to support that CBD acts as an antagonist at CB1R and CB2R. Although CBD has been determined to have low affinity for both of these receptors, it has exhibited high potency.30 The fact that THC exhibits partial agonist activity at CB1R whereas CBD exhibits antagonist activity at this receptor exemplifies the distinct differences involved with these two phytocannabinoids and their activities on receptors in the brain. Considering that THC and CBD both possess antiepileptic properties while exerting different effects on neuronal activity creates additional questions related to their clinical use in epilepsy. It may be that when THC and CBD are both used together they may provide benefit, however the ratios in relation to each other and dosing parameters are not clearly defined. Additionally, both cannabinoids have activity beyond CB1R and CB2R. It has been suggested that CBD may exhibit anti-epileptic properties as a result of multiple interactions with other receptors and ion channels.27, 31 CBD is speculated to have effects on receptors such as transient receptor potential channels, PPARϒ, GPR55, GPR18, 5HT1A and VDAC1.31, 32 When GPR55 is agonized in the brain, it mobilizes intracellular calcium elevating the presynaptic amount of calcium resulting in glutamate release.27 Interestingly, CBD is a GPR55 antagonist which would aid in glutamate release inhibition, thus decreasing the rate of the neuronal action potentials and seizure activity.27 The voltage-dependent anion channel protein (VDAC1) is a major component of the mitochondria membrane that regulates ion exchange.32 Studies propose that CBD helps to regulate the homeostasis of calcium in the mitochondria, which prevents calcium fluctuations under high excitability conditions.31, 33 It has also been suggested that CBD inhibits the reuptake and degradation of AEA by inhibiting FAAH and therefore increasing AEA’s agonistic effects on CB1R.31, 34, 35 Studies have shown that CBD works in a bi-phasic fashion demonstrating that its actions are heavily dose dependent.31 The possible synergistic or inhibitory activity with other phytocannabinoids found in marijuana such as THC are also important considerations. The inhibitory action produced by THC at CB1R offers a mechanism to diminish neuronal excitability, but at the cost of psychoactive properties and cognitive impairment. CBD’s actions provide a possible mechanism for inhibition of excitatory neurons (through different pathways involving GPR55 and resulting increased levels of AEA), but without the adverse effects mentioned above. Although there is supportive research that analyzes specific pathways for neuronal activity modulation using endocannabinoids, the full extent of each phytocannabinoid’s role is not clear. Additional studies are needed that focus on the variety of constituents and various concentrations from different Cannabis plants, strains and formulations.
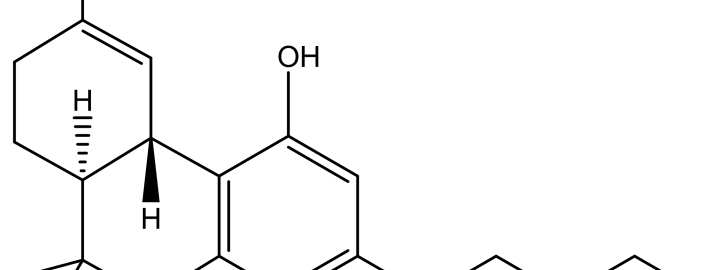
The structures of cannabinoids present in Cannabis (Figures 1 and 2) as well as the endogenous ligands of CB1R and CB2R (Figures 3 and 4) are provided. The structures of the Cannabis cannabinoids are quite similar to each other and this is also true of the endogenous cannabinoids. Interesting, however, is that the two separate families of cannabinoids lack structural similarity to one another. Structure is a key determinant of binding affinity, pharmacodynamics and pharmacokinetic profiles and this highlights the importance of emphasizing research that establishes ADME profiles, efficacy, potency, and clearance parameters under a variety of conditions and strategies of formulation.
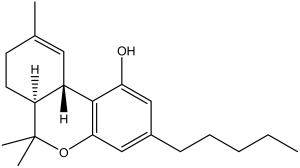
Figure 1. Delta (9)-tetrahydrocannabinol (THC)
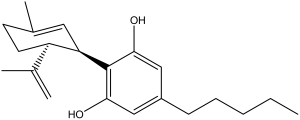
Figure 2. Cannabidiol (CBD)
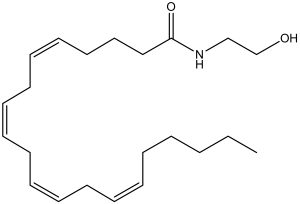
Figure 3. Anandamide
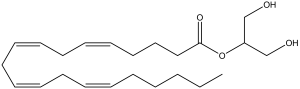
Figure 4. 2-arachidonoylglycerol
Currently available formulation of marijuana utilized for the treatment of epilepsy
Cannabis can be administered by inhalation, oral ingestion and topically. There is large variation in pharmacokinetic parameters depending on the route of administration. The inhalation route, either by smoking or vaporization provides the fastest onset, however lung administration burns lung tissue via direct smoke or increased temperatures with vaporization. Oral administration is associated with inconsistent absorption and onset of action. Gastric contents and first pass metabolism affect systemic absorption and dosing adjustments are recommended in the presence of food. Onset after oral administration can vary from 30 minutes to 2 hours or longer, with duration of action ranging from 5 to 8 hours.36 Little data is available on the pharmacokinetics of topical administration of cannabinoids and, to date, there are not any studies researching this formulation strategy for the use in epilepsy.36
In the state of Colorado an oil based formulation, known as Charlotte’s WebTM, is available from a non-profit organization “Realm of Caring” for the treatment of seizures in addition to other medical conditions.37 There is currently a waitlist for this formulation and patients and caregivers are required to move to Colorado in order to obtain this medical formulation of Cannabis. The formulation is stated to be highly concentrated in CBD, with less than 0.3% of THC, providing a formulation not likely to cause psychoactive activity as result of the low concentration of THC.37 Currently there are not any clinical trials evaluating the efficacy of this product, however this non-profit organization is currently working on observation trials.37
Conclusion
It is exciting to consider the possibilities that Cannabis may provide for treatment options in epilepsy. It is also evident that additional studies are needed to better understand the dynamic way in which different components of marijuana may play a role in preventing seizure activity in patients diagnosed with epilepsy. In addition, the pharmacokinetic parameters most likely to achieve therapeutic benefit without adverse effects and drug interactions require further investigation. Research is also needed to evaluate the specific metabolism of CBD and the optimization of patient centered dosing of these highly concentrated CBD formulations. Despite the need for additional research, current and ongoing clinical trials provide strong evidence to support the use of Cannabis formulations in epilepsy. This is especially true for those that are unable to achieve therapeutic success with currently available AED medications.
References
- Amtmann D, Weydt P, Johnson KL, Jensen MP, and Canter GT. Survey of cannabis in use in patients with amyotrophic lateral sclerosis (ALS). American Journal Hospital Palliative Care.2004;21(2): 95-104.
- Koppel BS, Brust JC, Bronstein J, Youssof S, Gronseth G and Gloss D. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology.Neurology.2014;82(17): 1556-63.
- Syed YY, McKeage K, and Scott LJ. Delta-9-tetrahydrocannabinol/cannabidiol (sativex(®)): a review of its use in patients with moderate to severe spasticity due to multiple sclerosis.Drugs.2014;74(5): 563-78.
- Institute of Medicine. 2012. Epilepsy across the spectrum. March 30. Accessed July 16, 2014. http://www.iom.edu/Reports/2012/Epilepsy-Across-the-Spectrum.aspx.
- Cunha JM, Carlini EA, and et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients.Pharmacology.1980;21(3):175-85.
- Ames FR, and Cridland S. Anticonvulsant effect of cannabidiol. S Afr Med J.1986;4(1):14.
- Elger CE, Schmidt D. Modern management of epilepsy: A practical approach. Epilepsy & Behavior.2008;12(4):501-39.
- Jefferys, JG. Models and mechanisms of experimental epilepsies. Epilepsia.2003;(12): 44-50.
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Hauser WA, Mathern G, Moshé SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on therapeutic strategies. Epilepsia. 2010;51(6):1069-1077.
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319.
- Kong ST, Ho CS, Ho PC, Lim SH. Prevalence of drug resistant epilepsy in adults with epilepsy attending a neurology clinic of a tertiary referral hospital in Singapore. Epilepsy Res. 2014;108(7):1253-1262.
- Feldmann M, Asselin MC, Liu MC, Wang S, McMahon A, Anton-Rodriguez J, Walker M, Symms M, Brown G, Hinz R, Matthews J, Bauer M, Langer O, Thom M, Jones T, Vollmar C, Duncan JS, Sisodiya SM, Koepp MH. P-glycoprotein expression and function in patients with temporal lobe epilepsy: a case-control study. Lancet Neurol. 2013;12(8):777-785.
- Loup F, Picard F, Yonekawa Y, Wieser HG, fritschy JM. Selective changes in GABAA receptor subtypes in white matter neurons of patients with focal epilepsy. Brain.2009;132(pt 9):2449-2463.
- He X, Li Y, Liu Z, Yue X, Zhao P, Hu J, Wu G, Mao B, Sun D, Zhang H, Song X, Wang Y, Shao J. The association between CCL2 polymorphisms and drug-resistant epilepsy in Chinese children. Epileptic Disord. 2013;15(3):272-277.
- Kudin AP, Zsurka G, Elger CE, Kunz WS. Mitochondrial involvement in temporal lobe epilepsy. Exp Neurol. 2009;218(2):326-332.
- Alonso-Vanegas MA, Cisneros-Franco JM, Castillo-Montoya C, Martínez-Rosas AR, Gómez-Pérez ME, Rubio-Donnadieu F. Self-reported quality of life in pharmacoresistant temporal lobe epilepsy: correlation with clinical variables and memory evaluation. Epileptic Disord. 2013;15(3):263-271.
- Tellez-Zenteno JF, Ronquillo LH, Wiebe S. Sudden unexpected death in epilepsy: evidence-based analysis of incidence and risk factors. Epilepsy Res. 2005;65(1-2):101-115.
- Surges R, Adjei P, Kallis C, Erhuero J, Scott CA, Bell GS, Sander JW, Walker MC. Pathologic cardiac repolarization in pharmacoresistant epilepsy and its potential role in sudden unexpected death in epilepsy: a case-control study. Epilepsia. 2010;51(2):233-242.
- Heuser K, Szokol K, Taubøll E. The role of glial cells in epilepsy. Tidsskr Nor Laegeforen. 2014;134(1):37-41.
- Liu X, Liu J, Liu J, Liu XL, Jin LY, Fan W, Ding J, Peng LC, Wang Y, Wang X. BDNF-TrkB signaling pathway is involved in pentylenetetrazole-evoked progression of epileptiform activity in hippocampal neurons in anesthetized rats. Neurosci Bull. 2013;29(5):565-575.
- Vezzani A, Balosso S, Maroso M, Zardoni D, Noé F, Ravizza T. ICE/caspase 1 inhibitors and IL-beta receptor antagonists as potential therapeutics in epilepsy. Curr Opin Investig Drugs. 2010;11(1):43-50.
- McPartland JM, Guy GW, Di Marzo V. Care and feeding of the endocannabinoid system: a systematic review of potential clinical interventions that upregulate the endocannabinoid system.” PLOS ONE.2014;9(3): e89566.
- Fezza F,Marrone MC, Avvisati R, Di Tommaso M, Lanuti M, Rapino C, Mercuri NB, Maccarrone M, and Marinelli S. Distinct modulation of the endocannabinoid system upon kainic acid-induced in vivo seizures and in vitro epileptiform bursting. Molecular and Cellular Neuroscience.2014;62C:1-9.
- Pertwee RG. Targeting the endocannabinoid system with cannabinoid receptor agonists. Philos Trans R Soc Lond B Biol Sci.2012;367(1607):3353-63.
- Dlugos A, Childs E, Stuhr KL, Hillard CJ, and de Wit H. Acute stress increases circulating anandamide and other N-Acylethanolamines in healthy humans. Neuropsychopharmacology.2012;37(11): 2416-2427.
- Skaper SD, Di Marzo V. Endocannabinoids in nervous system health and disease: the big picture in a nutshell. Philos Trans R Soc Lond B Biol Sci.2012;367(1607):3193-200.
- Sylantyev S, Jensen TP, Ross RA, and Rusakov DA. Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc Natl Acad Sci U S A.2013;110(13): 5193-5198.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. British Journal of Pharmacology.2008;153(2): 199-215.
- Hofmann ME, Frazier CJ. Marijuana, endocannabinoids, and epilepsy; potential and challenges for improved therapeutic intervention. Experimental Neurology.2013;244: 43-50.
- Thomas A., Baillie GL, Phillips AM, Razdan RK, Ross RA, and RG Pertwee. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro.Br J Pharmacol. 2007;150(5):613-23.
- Cilio MR, Thiele EA, Devinsky O. The case for assessing cannabidiol in epilepsy. Epilepsia. 2014;55(6):787-90.
- Rimmerman, N., D. Ben-Hail, Z. Porat, A. Juknat, E. Kozela, MP. Daniels, PS. Connelly, E. Leishman, HB. Bradshaw, V. Shoshan-Barmatz, and Z. Vogel. 2013. Direct modulation of the outer mitochondria membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: A novel mechanism for cannabinoid-induced cell death.” Cell Death Dis. 20135;4:e949.
- Ryan D, Drysdale AJ, Lafourcade C, Pertwee RG, Platt B. Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J Neurosci. 2009;18;29(7):2053-63.
- Ryan D, Drysdale AJ, Pertwee RG, Platt B. Interactions of cannabidiol with endocannabinoid signaling in hippocampal tissue. Eur J Neurosci. 2007;25(7):2093-102.
- Fernandez-Ruiz J, Sagredo O, Pazos MR, Garcia C, Pertwee R, Mechoulam R, Martinez-Orgado J. Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br J Clin Pharmacol. 2013;75(2):323-33.
- Aggarwal SK. Cannabinergic pain medicine: a concise clinical primer and survey of randomized-controlled trial results. Clin J Pain. 2013;29(2):162-71.
- Realm of Caring. 2014. FAQ General: Realm of Caring. Accessed September 13, 2014. http://theroc.us/index.php?option=com_content&view=article&id=77&Itemid=427.